WATCHMAN FLX™
Kategori ID: 4
Açıklama: ush forward to the future of LAAC. Confidently. WATCHMAN FLX is the new generation LAAC device combining the experience of a clinically proven platform with a new, intuitive implantation technique for confident LAA closures of even challenging anatomies due to its excellent conformity to LAA anatomies.
Push forward to the future of LAAC. Confidently.
THE FEEL.
Full control for an intuitive, safe and precise positioning
THE SEAL.
Enhanced conformability for confident closure
THE HEAL.
Minimal metal exposure for optimized healing
WATCHMAN FLX
Product Animation
Watch the animation now
A journey of success
WATCHMAN FLX received the CE mark in February 2019 and more than 2.500 patients benefitted in 2019 from this new technology. Discover the key milestones and clinical evidence of the next generation LAAC device.
Join us on the FLX journey
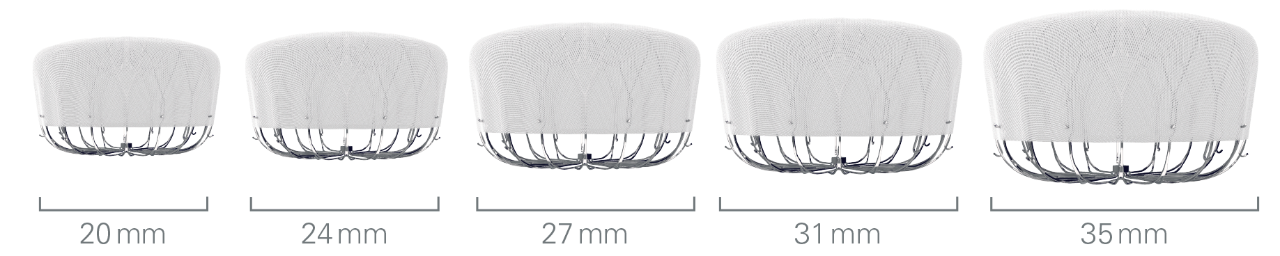
Flexibility to expand treatment options
The WATCHMAN FLX™ device comes in five sizes and can treat ostia from 14mm to 31.5mm.
Product Information
| Description | Specifications |
| WATCHMAN FLX™ | Nitinol frame with Polyethylene Terephthalate (PET) with distal fluoroscopic marker |
Delivery Catheter
| Description | Specifications |
| Sheath Material | Braided Pebax® with PTFE liner and platinum/iridium marker band |
WATCHMAN TruSeal Access Sheath
| Description | Specifications |
| Hub | Material Pebax® with polycarbonate cap |
| Sheath Material | Pebax® with PTFE liner and platinum/iridium marker band |
| Dilator | HDPE/LDPE high density polyethylene/low density polyethylene (50:50 blend) |
WATCHMAN FLX is preloaded into the delivery catheter, reducing preparation time
All cited trademarks are the property of their respective owners.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Information for use only in countries with applicable health authority registrations. Material not intended for use in France.

