Relianceᵀᴹ 4-Front
Kategori ID: 5
Açıklama: RELIANCE 4-FRONT leads are 7.3F (2,4 mm), steroid-eluting, endocardial cardioversion/defibrillation and pace/sense leads. 4-FRONT is built on the reliable RELIANCE™ platform and has a smart size reduction while maintaining insulation thickness. Key Resource
Product Specifications
Lead Specifications
RELIANCE 4-Front™, Single-coil
RELIANCE 4-Front™, Dual-coil
The RELIANCE 4-FRONT leads are 7.3F (2.4 mm) (8F/2.6 mm introducer), steroid-eluting, endocardial cardioversion/defibrillation and pace/sense leads available in extendable/retractable models as well as in passive fixation models. These leads utilize the DF4 connector and incorporate the IROX™ (iridium oxide) coating. The silicone lead body has a lubricious coating and the electrode coils are backfilled with silicone.
| Product | Dual-coil Active | Single-coil Active | Dual-coil Passive | Single-coil Passive |
|---|---|---|---|---|
| Model/Length | 0675 59 cm 0676 64 cm |
0672 59cm 0673 64cm |
0665 59 cm 0636 64 cm |
0662 59 cm 0663 64 cm |
| Terminal Configuration1 | DF4-LLHH | DF4-LLHO2 | DF4-LLHH | DF4-LLHO |
| PG Compatibility | RELIANCE 4-FRONT leads with the DF4-LLHH / LLHO label are equivalent and are compatible with a device containing either a GDT-LLHH or DF4-LLHH port | |||
| Lead Introducer without Guide Wire | 8F⁴ (2.6 mm) | 8F⁴ (2.6 mm) | 8F⁴ (2.6 mm) | 8F⁴ (2.6 mm) |
| Lead Introducer with Guide Wire | 10.5F (3.5 mm) | 10.5F (3.5 mm) | 10.5F (3.5 mm) | 10.5F (3.5 mm) |
| Isodiametric Lead Body Diameter | 7.3F (2.4 mm) | 7.3F (2.4 mm) | 7.3F (2.4 mm) | 7.3F (2.4 mm) |
| Rotations Expected to Extend/Retract Helix3 | 11 | 11 | n/a | n/a |
| Tip/Helix Electrode Surface Area (mm2) | 5.7 | 5.7 | 3.5 | 3.5 |
| Proximal Coil Active Electrode Surface Area (mm2) | 660 | n/A | 660 | n/a |
| Distal Coil Active Electrode Surface Area (mm2) | 450 | 450 | 450 | 450 |
| Tip to Proximal Coil Electrode Length (mm) | 180 | n/a | 180 | n/a |
| Tip to Distal Coil Electrode Length (mm) | 12 | 12 | 12 | 12 |
| Lead Body Insulation Material | layer of silicone, layer of polyurethane (for the first ~ 12 cm) and then the silicone trilumen | |||
| Terminal Pin Material | MP35N nickel-cobalt alloy | |||
| Pace/Sense Conductor Material | low titanium, MP35N nickel-cobalt alloy, PTFE sleeve | |||
| Conductive Ring Material | MP35N nickel-cobalt alloy | |||
| Shocking Conductor Material | 1X19 Low titanium MP35N nickel-cobalt alloy, silver-core, drawn filled tube, ETFE coated | |||
| Tip Electrode Material | IROX coated platinum / iridium | |||
| Coil Electrode Covering Material | Silicone | |||
| Shock Coil Material | Platinum clad tantalum clad titanium | |||
| Steroid Material | Approximately 0.96 mg dexamethasone acetate nominally | |||
Features
Lifetime Warranty: The RELIANCE 4-FRONT defibrillation lead family is backed with a lifetime warranty5.
Terminal configuration: The RELIANCE 4-FRONT lead is DF4-LLHH for dual-coil leads and DF4-LLHO for single-coil leads, this suffix provides functional identifications of conductors:
L = Low Voltage
H = High Voltage
O = Inactive Ring Contact (Single-Coil Leads Only)

Rings 1 and 2 are electrically connected within the terminal for integrated bipolar pacing / sensing. Cable conductors
are utilized for both shock coils. Ring 2 is connected to the distal shock coil and ring 3 connects to the proximal coil.
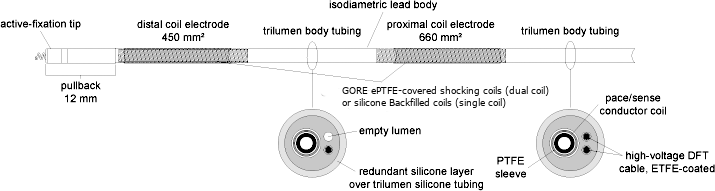
Isodiametric lead body:
The isodiametric lead body contains one conductor for pacing / sensing. For defibrillation, the lead has two conductors in dual coil models and one for the single coil models leaving one lumen empty in single coil models. The conductors are insulated in separate lumens within the silicone rubber lead body. A second layer of silicone covers the lead body, providing additional insulation and a uniform body diameter. RELIANCE 4-FRONT has a 7.3F (2.4 mm) lead body which fits through an 8F (2.6 mm) non-hemostatic introducer when not retaining a guide wire.
Insulation:
Silicone construction: Silicone has been used in Boston Scientific leads for nearly 4 decades.
Polyurethane sleeve: The first 12 cm of the lead distal to the terminal boot incorporates a polyurethane sleeve underneath the outer silicone rubber insulation for enhanced abrasion resistance within the pocket.
Lubricious coating: The RELIANCE 4-FRONT lead family utilizes a proprietary coating that makes the silicone lead surface more lubricious. This reduces both the static and dynamic coefficients of friction, making the lead surface feel and handle like polyurethane while providing the time-tested reliability of silicone.
Silicone backfilled coil: is defined as a process where the coils are coated with silicone, cured for a short period of time and the silicone is wiped off from the topsurface of the filars, leaving silicone between the filars to mitigate tissue ingrowth
IROX coating: RELIANCE 4-FRONT features an IROX coated pace/sense cathode electrode, which may improve pacing performance. Lower and more predictable pacing thresholds may increase the longevity of the pulse generator.
Steroid distal tip: The tip electrode contains a nominal dose of steroid that elutes upon exposure to body fluids. The steroid suppresses the inflammatory response believed to cause threshold rises typically associated with implanted pacing electrodes. Lower thresholds are desirable because they can increase pacing safety margins and reduce pacing energy requirements, potentially increasing pulse generator longevity.
Pullback: Pullback is the distance the defibrillation electrode is removed from the lead tip, a critical factor in helping to direct energy deep into the ventricular apex. Standard for multiple generations of Boston Scientific defibrillation leads, the 12 mm RELIANCE 4-FRONT pullback design is important for low defibrillation thresholds, while optimizing sensing characteristics.
Radiopaque suture sleeve: The radiopaque suture sleeve is visible under fluoroscopy and is used to secure and protect the lead at the venous entry site after lead placement. The window feature is designed to aid compression of the sleeve onto the lead during suturing.
Passive-Fixation Features

Design leveraged from successful FINELINE 2 family. 12 mm Tip to RV Coil spacing is identical to RELIANCE.
Incorporates a flexible neck region and IROX coating for improved pacing performance.
Active-Fixation Features
Terminal pin-driven extendable/retractable fixation helix: Rotating the knob of the EZ-4 Connector tool rotates the terminal pin which extends / retracts the helix. The IROX coated platinum-iridium helix anchors the pacing electrode to the endocardial surface without support of trabecular structures, offering various lead placement possibilities for the tip electrode in the right ventricle.
Fluoroscopic markers: The RELIANCE 4-FRONT active fixation model incorporates a radiographic marker system to enable clear visualization of the helix position under fluoroscopy.
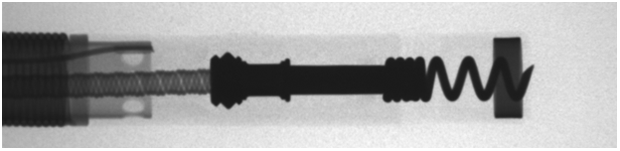
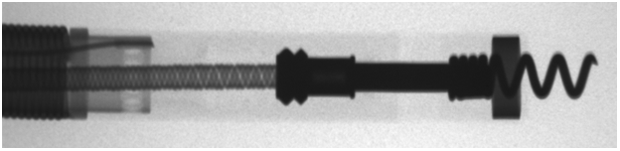
Mapping: The RELIANCE 4-FRONT tip and helix design allows mapping even with the helix fully retracted. Helix is flush to prevent snagging while enabling mapping.
EZ-4 ™ Connector Tool

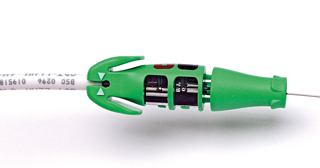
When connected to the lead, the EZ-4 Connector Tool performs the following functions:
1. Protects the lead terminal during the implant procedure.
2. Provides a safe and secure connection between the pacing system analyzer (PSA) patient cables and
the lead terminal.
3. Guides the stylet into the lead through the stylet funnel.
4. For leads with an extendable / retractable helix, rotates the terminal pin clockwise or counterclockwise to extend or retract the helix.
The EZ-4 Connector Tool is intended to be left on the lead for the duration of the implant, until the lead terminal is inserted into the header.
Key Benefits
RELIANCE 4-FRONT is the latest in ICD lead technology, built on the proven RELIANCE platform to provide:
- The most reliable lead on the market6
- A modest size reduction
- An improved implant experience
- Enhanced extraction capability
1. Conforms to ISO 27186:2010 standards 2. DF4-LLHO leads are compatible with a device DF4-LLHH port
3. Use the fluoroscopy markers for verification of full helix extension/retraction
4. When retaining a guide wire, a 2.5F (0.8 mm) increase in introducer size is recommended
5. Limited lifetime warranty. For a full and complete description of the RELIANCE 4-FRONT
6. Product Performance report 2020 Q3 Edition (99.6% survival probability at 6 years)
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device. or at www.IFU-BSCI.com.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.
This material not intended for use in France.
